
Genetic testing is an effective tool for preventing genetic diseases in many dog breeds. Genetic testing can also be used for known diseases and traits in the Australian Shepherd. Testing is recommended for both male and female dogs prior to planned mating. In the next section of this article you will find a description of the genetic diseases in the Australian Shepherd that are included in our package Australian Shepherd PANEL | GenoCan.eu.
Malignant hyperthermia (MH) – Malignant hyperthermia is a rare disease in normal life without symptoms, but serious complications occur only during anaesthesia for some of the drugs used (e.g. halothane, isoflurane or sevoflurane), and which can be fatal. MH is a disease of skeletal muscle in which affected individuals have no clinical symptoms until they are exposed to triggers, in this case anaesthetics. It involves prolonged muscle contraction without relaxation and overproduction of heat (hyperthermia), muscle rigidity and tachycardia. Complications require discontinuation of anaesthesia, cooling of the body and administration of antidotes, substances that reduce muscle tension. If anaesthesia is not discontinued, arrhythmias, rhabdomyolysis, renal failure and death can occur. Alternative anaesthetics may be chosen for dogs with MH.
MH has been described in humans and various species of animals such as dogs, horses or pigs. In dogs, the inheritance is autosomal dominant, where a single copy of the gene is sufficient for the disease to manifest in a given individual and the risk of passing the disability to offspring is 50%.
Degenerative myelopathy (DM)
Degenerative myelopathy is an incurable, progressive disease that occurs in a wide range of dog breeds with onset of symptoms usually around the age of eight and with equal frequency in both sexes. As the dog gets older, incoordination of movements worsens, followed by muscle atrophy, ataxia, incontinence, and inability to move the pelvic limbs associated with spasm leading to complete loss of pelvic mobility.
The enzyme superoxide dismutase 1, which is present in cells and is an important antioxidant, is considered to be an important factor in the development of the disease. If it is dysfunctional, it can accumulate in cells and even be toxic.
DM is an autosomal recessive disease with variable penetrance and occurs in individuals who inherit the mutated gene from both parents. Very rarely, the disease can occur in heterozygous individuals (carriers). At the same time, a dog with both mutant alleles may not get the disease and is only at high risk of developing the disease. Time and severity of the disease of the disease cannot be determined and vary.
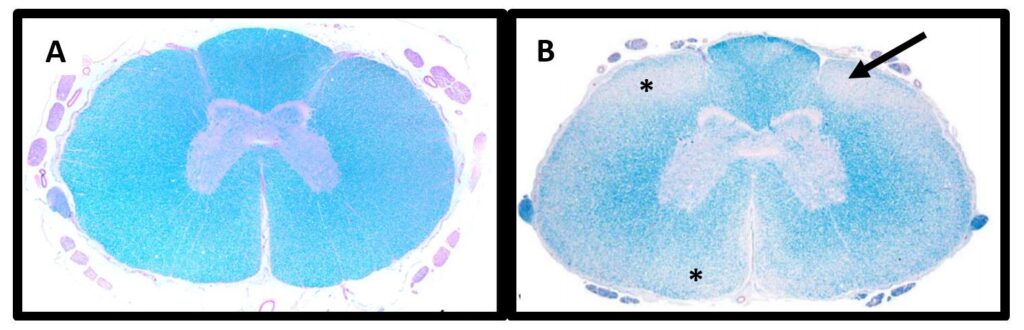
Reference: Awano T, et al. PNAS (2009). 106(8):2794-2799 and March PA, et al. Vet Path (2009). 46:241-250.
B-Locus – Red colour

In the inheritance of colours, the hierarchy and interaction of the most important genes is described. In addition to the major genes MC1R (locus E) and ASIP (locus A) for the synthesis of eumelanin (brown and black pigment) or pheomelanin (light and red pigment), several other genes have been identified in relation to coat colour in dogs, e.g. TYRP1 (locus B, for black or brown to red colour), MLPH (locus D, dilutes dark pigment), MFSD12 (diluting locus I to white coat colour), etc.
The black colour of the coat and nose is determined by the dominant allele B, i.e. the presence of only one of these alleles is sufficient (genotype B/B or B/b). For red colouration, both recessive b alleles are needed (genotype b/b). Three variants of the TYRP1 gene were detected: c.991C>T (bs), c.1033_1036delCCT (bd) and c.121T>A (bc). Only in the Australian Shepherd breed a fourth variant was identified: baus c.555T>G.
If an individual carries the variants bc/bc or bd/ bd or bs/bs, the individual should be red in the typical places for the breed. However, if it carries bc / bs or bc / bd or bd / bs, it is not possible to describe the genotype for the B-locus without testing the parents, as it is not possible to distinguish whether the variants are on one copy of the gene or both (e.g. it is not possible to distinguish B / bc+bs from bc / bs).
Hereditary cataract (HC-HSF4)
Hereditary cataract (cataracts) is a disease affecting the lens of the eye in which the lens gradually becomes cloudy and is one of the most important causes of blindness in dogs.
The primary hereditary form of cataract affects many breeds of dogs, with particular differences between breeds in the age of onset, rate of progression, degree of bilateral symmetry, and location of the cataract in the lens, but the mode of inheritance of cataract may also vary between breeds. Conversely, within a breed, hereditary cataracts usually show very similar clinical manifestations.
Only in the Staffordshire Bull Terrier, the Boston Terrier, the French Bulldog and the Australian Shepherd has the main genetic mutation responsible for hereditary cataract been identified. In the first three breeds, the mutation is inherited in an autosomal recessive manner and clinical signs appear within the first year of life. Whereas in Australian Shepherds, a different mutation of the same gene occurs and is inherited in an autosomal dominant manner. However, all carriers of the deletion may not have an outbreak of the disease, i.e. it is an incomplete penetrance.
Multiple Drug Resistance (MDR1)
Multiple Drug Resistance is an inherited disease in Collies, Shelties, Australian Shepherds, Bobtails, Border Collies and many others that causes affected dogs to be extremely sensitive to certain drugs (ivermectin, loperamide and others). Exposure to these groups of drugs can lead to severe neurological symptoms such as hypersalivation, ataxia, blindness, tremors, respiratory problems and sometimes death.
The cause of the disease is a dysfunctional P-glycoprotein. It is responsible for transporting drugs and toxic substances from the brain into the blood and plays a key role in breaking down these drugs. When this protein is dysfunctional, the levels of these substances rise, resulting in a neurotoxic reaction.
The disease is inherited in an autosomal recessive pattern, which means that affected individuals must inherit a mutation on both copies of the gene. It is known that even carriers can have a mild adverse reaction after administration of these drugs.
Progressive rod and cone degeneration (PRA-prcd)
Progressive retinal atrophy, progressive rod degeneration (PRA-prcd) is a hereditary late-onset eye disease affecting many breeds of dogs. PRA-prcd results from degeneration and death of the retinal lens cells (rods and cones). At first the rods lose their function, and over time affected dogs lose their night vision. Then the cones begin to degenerate and dogs begin to show visual disturbances in bright light. Although there are individual and breed differences in the age of onset and rate of progression of the disease, in most dogs the disease eventually results in complete blindness.
PRA-prcd is autosomal recessive and will only manifest itself in individuals who have inherited the mutated gene from both of their parents (recessive homozygotes).
Collie Eye Anomalies (CEA)
According to its name, it is a disease affecting collies, but it mainly affects sheepdog and herding breeds such as shelties, border and bearded collies, Australian Shepherds and many others. Already during embryonic development there is a defective, imperfect formation (hypoplasia) of the choroid or retina. CEA is observable near the optic disc as a lighter or thinner area on ophthalmic examination and can be diagnosed in young puppies. The most appropriate time for their examination is 7-8 weeks of age. Later (after 3 months of age), ophthalmic examination might not show the presence of the disease. Diagnosis may be more difficult in dogs with “blue merle” colouration, where the inside of the eye may appear lighter on eye examination.
The defect is autosomal recessively inherited, meaning that only individuals who inherit the mutated gene from both parents will develop the disease.
Hyperuricosuria (HUU)
Hyperuricosuria is a waste protein excretion disorder mainly in Dalmatians, but has also been described in Australian Shepherds, English Bulldogs, Black Russian Terriers, Pomeranians, American Staffordshire Terriers, German Shepherds, Great Schnauzers, Parsons and Jack Russell Terriers, Boerboels and Weimaraners. This condition predisposes affected individuals to the formation of urinary stones.
In a healthy dog, purines ingested in the diet are converted in the liver to uric acid, which is then converted to allantoin, which is soluble and excreted in the urine. In the affected individual, most of the uric acid remains unchanged. A high level of uric acid in the urine does not itself cause problems, but if it crystallises, urinary sand or stones are formed. Hyperuricosuria is inherited in an autosomal recessive manner, meaning that only individuals who inherit the mutated gene from both parents will develop the disease.
Multifocal retinopathy type I (CMR1)
Multifocal retinopathy type 1 is a genetic eye disorder that manifests as degeneration of the retina, which can lead to progressive loss of vision up to complete blindness. This disease occurs in a variety of dog breeds. The development of the disease is very rapid, often appearing before the age of four months. The first manifestations are in the form of lesions under the retina, similar to vesicles. Despite gradual damage to the retina, complete loss of vision usually occurs only at an older age.
The protein bestrophin is responsible for the proper assembly of the retinal pigment epithelium. If it is dysfunctional, it causes atrophy of the retinal pigment epithelium and leads to severe impairment of central vision. Inheritance is autosomal recessive, meaning that the disease develops only in individuals who inherit the mutated gene from both parents.
Neuronal Ceroid Lipofuscinosis (NCL6)
Neuronal ceroid lipofuscinosis is a very rare neurodegenerative disease characterized by the accumulation of lipid wastes in nerve cells. Specifically, the NCL6 type has been reported in Australian Shepherds. The onset of the disease occurs at approximately one and a half years of age. Symptoms may include loss of vision, behavioural changes (unreasonable expressions of fear and anxiety, uncertainty of movement even in familiar surroundings, demented behaviour, hyperactivity or rage), impaired motor and cognitive abilities, and epileptic-like seizures. The disease ends in the premature death of the affected individual, usually within one year after the first signs of the disease appear.
It is an autosomal recessive inheritance and only occurs in individuals who have acquired the mutated gene from both parents.
Primary Ciliary Dyskinesia in Australian Shepherds (PCD)
Primary Ciliary Dyskinesia is a rare disease in which the cilia in the airways do not fully function. This respiratory disease is characterized by recurrent inflammation of the upper and lower airways that requires repeated treatment. Clinical signs begin to appear in puppies.
Inheritance is autosomal recessive, i.e. the disease develops only in individuals who inherit the mutated gene from both parents.

Short-tail (Bob-tail, NBT)
This mutation naturally produces Natural Bob tail (NBT), i.e. stunting of the tail vertebrae. This pattern is dominant and will always show up on the individual, but can vary in length. From almost long, to half, ¼, to completely short. There can be a whole range of these lengths in one litter.
Short tail is caused by the c.189C>G mutation in the TBXT gene. This mutation has been shown to be heterozygous. The homozygous condition is lethal at the embryonic stage due to severe spina bifida. When two heterozygous individuals were crossed, a 30% loss of puppies in the litter was demonstrated.




